Reverse Osmosis
Osmosis is a natural phenomenon, which takes place when two solutions at different concentrations are separated by a semi permeate membrane. Under this condition, pressure is created which forces the solvent to pass through the membrane from diluted solution to concentrated solution. This movement of pure solvent is called osmosis and a pressure at which it occurs is called osmotic pressure. When a Pressure is applied gradually to the concentrated solution, a stage is reached when there is no flow of pure solvent through the membrane and as, we apply excess pressure the pure solvent starts flowing through the membrane from concentrated solution to the diluted solution. This movement of pure solvent separation from the concentration solution is called reverse osmosis. The levels of most dissolved compounds and membrane type and operating conditions will also affect the degree of pollutant removal.
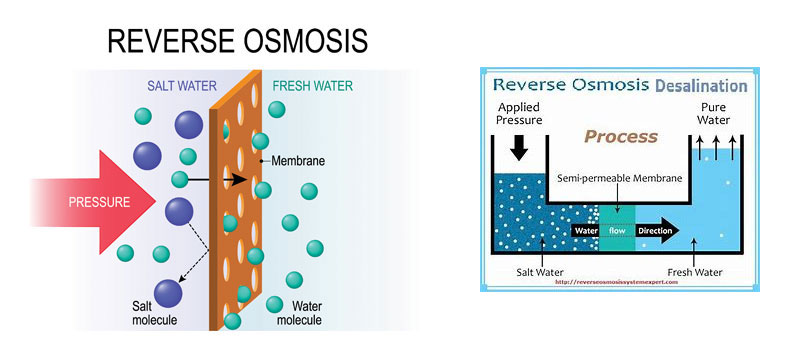
Reverse Osmosis Desalination Features:
2.1 Sea Water Reverse Osmosis Plant-Typical Flow Chart

2.2 Brackish Water Reverse Osmosis Plant-Typical Flow Chart

